Apoptosis, or Programmed Cell Death, is key to
multicellular life and multicellular computing
Multicellular organisms protect and even "sculpt" themselves by programmed cell suicide -- a process called apoptosis
Of the four principles of multicellular systems, perhaps the least obvious is apoptosis (sometimes spelled aptosis or apotosis, and also known as Programmed Cell Death or PCD). Simply put, the principle is that all of a multicellular organism's cells are prepared to suicide when needed for the benefit of the organism as a whole. They eliminate themselves in a very carefully programmed way so as to minimize damage to the larger organism. Moreover, they don't do it only when things go wrong! The apoptosis mechanism is a normal and creative aspect of multicellular life. Orchestrated apoptosis helps the growing embryo to sculpt many aspects of its final form. It is also a part of normal "maintenance." Every year the average human loses half of his/her body weight in cells via apoptosis! And apoptosis protects the organism from "rogue" cells because such cells self-destruct when their internal mechanisms go wrong except when the apoptosis mechanism itself is compromised, as happens in the development of cancer.
Because apoptosis is so crucial to the growth and survival of multicellular organisms, it is carefully intertwined with the other three multicellular principles.
Now that "viruses," "worms" and the like bedevil the Internet, we need an analogous principle of computing apoptosis to keep infected computers from infecting others. Computers need to be able to recognize their own infection and shut down their connection to other computers whether on the Internet or on a local wired or wireless network.
What triggers cellular apoptosis?
No sooner has a Metazoan (i.e., multicellular organism) begun to organize itself, than it is subject to the slings and arrows of fate. Constituent cells can become infected with viruses or attacked by bacterial predators, their DNA can be damaged by replication errors, radiation or mutagenic chemicals. Or cells can lose their differentiation and thereby become neoplastic and eventually cancerous. If a cell is infected it may, in turn, infect its neighbors. DNA replication errors or mutations will be passed on to the cell�s progeny. Cancer cells, of course, grow rapidly without bound and disrupt vital bodily functions.
Metazoan cells have evolved mechanisms for detecting internal errors or external assaults that might threaten the whole organism. When such threats are detected, the cells react by killing themselves. For many types of cell, loss of contact to the extracellular matrix (which is the body's stigmergy structure) triggers apoptosis. That is, if the cell somehow becomes detached from its proper place in the body, it self-destructs.
Each cell in advanced Metazoans has two sorts of receptors on its surface that connect to the apoptosis mechanism. One type receives messages that inhibit apoptosis (think of them as "you're OK" messages) and the other type receives messages that encourage apoptosis (think of them as "you're not wanted" messages. Cell survival is determined by the balance between these two types of message.
Needless to say, the semantics of the �live or die� messaging mechanisms have evolved with care � all the more so because apoptosis is used not only to kill dangerous cells, but also to sculpt many body structures during development from an egg to an adult. For example, apoptosis causes the disappearance of a tadpole�s tail. At the appropriate stage of development of the frog, the balance between live and die messages to the cells in the tail shifts and causes the tail to self destruct in a very carefully orchestrated manner. Similar apoptosis mechanisms remove the webbing between embryonic human fingers so that the fingers can separate. Thus, from the perspective of the whole organism, apoptosis can be as much a creative process as a destructive one.
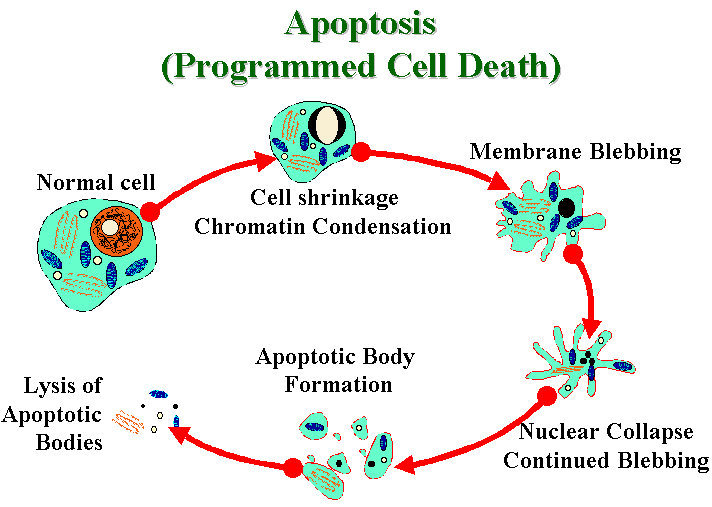 The apoptosis mechanism removes the cell with a
minimum of risk or damage to nearby cells. Apoptosis shrinks the
cell, degrades the DNA in its nucleus, breaks down the
mitochondria, and then breaks the cell into small, neat,
membrane-wrapped, fragments. Finally, nearby phagocytic cells
engulf the cell fragments. The phagocytic cells also secrete
cytokines that inhibit inflammation that would otherwise be a
danger to the surrounding cells.
The apoptosis mechanism removes the cell with a
minimum of risk or damage to nearby cells. Apoptosis shrinks the
cell, degrades the DNA in its nucleus, breaks down the
mitochondria, and then breaks the cell into small, neat,
membrane-wrapped, fragments. Finally, nearby phagocytic cells
engulf the cell fragments. The phagocytic cells also secrete
cytokines that inhibit inflammation that would otherwise be a
danger to the surrounding cells.
Apoptosis must remove cells in such a careful and well controlled manner because removing cells is just as important to the health of the multicellular organism as growing new cells.
Apoptosis is fundamental to multicellular life
Apoptosis evolved coincident with the first types of multicellular life: colonies of bacteria[1]. And �...the invention of apoptosis was an essential feature of the evolution of multicellular animals.[2]� Today all multicellular organisms, both plant and animal, rely on some form of programmed cell death. Apoptosis evolved to deal with the sorts of issues that face multicellular organisms but not single-cell organisms: initial development of the body, and after development, the maintenance of the organism against threats of DNA damage, viral infection, and loss of differentiation. Apoptosis solves those issues from a multicellular perspective. Not only have complex and specialized signaling mechanisms evolved so that each and every cell constantly determines whether it continues to be more valuable to the organisms alive than dead, but also once the decision has been made, it triggers a complex process that protects the whole organism. There can be no clearer demonstration of the value of multicellularity, i.e., the value in the cooperation of many different specialized elements. Neither the complex decision process nor the carefully staged suicide process are of use to single cell organisms.
It will become fundamental to multicellular computing too
As with multicellular biological organisms, apoptosis will necessarily become an important principle in multicellular computing. Computing can benefit from the two central lessons of apoptosis: first, the system must be architected so that no cell is indispensable and, second, maintaining the organisms is too important to be trusted to some "omniscient" centralized mechanism. Instead, the task of protecting the system must be given to every part of the system, including the very cells that may represent the threat. Protection is far more than putting up barriers, e.g., firewalls or anti-virus software; it also must include mechanisms to detect abnormal conditions and induce suicide.
This principle, unlike the other three principles of multicellularity, seems to fly in the face the common assumption that the way to build robust computing systems is to protect every computer at all cost. If we were able to protect every computer, well and good. But we are not. In fact, we seem to be losing ground in that effort. Apoptosis evolved in a world rife with viruses, mutations and other risks to biological organisms. Since modern computing systems now face similar challenges, a computing analog of apoptosis deserves careful consideration.
Once we incorporate an equivalent to apoptosis in computing, the creative aspect of apoptosis, i.e., its value in removing obsolete portions of the system, will also find a place in multicellular systems -- for example, to grow rapidly out to the boundary of a possible network, then cause elements that are not needed beyond the early growth stage to suicide.
[1] For example, modern cyanobacteria, the descendants of the earliest bacterial colonies, have an apoptosis pathway. See, �The demise of the marine cyanobacteria, Trichodesmium spp., via an autocatalyzed cell death pathway, Berman-Frank, I., Bidle, K.D., & Falkowski, P.G. Limnology and Oceanography, 49(4), pp. 997-1005, 2004. And E. coli, intestinal colony bacteria common to many mammals, especially humans, use an apoptosis-like mechanism to control their colony size in times of stress, see "Deadly Priming", R. Kolter, Science, vol 318, pp. 578-79, 26 Oct. 2007.
[2] Even the very simple Hydra uses apoptosis. See: �Identification of caspases and apoptosis in the simple metazoan Hydra,� Cikala M, Wilm B, Hobmayer E, Bottger A, David CN, Curr Biol., Sep 9;9(17):959-62, 1999.
Last revised 9/4/2013